Genetic architecture of Parkinson's disease: global initiatives and importance of underrepresented populations

Date: November 2022
Prepared by SIC Member: Mario Cornejo-Olivas, MD
Authors: Andrew Singleton, PhD; Cornelis Blauwendraat, PhD.; Ignacio F. Mata, PhD.
Editor: Lorraine Kalia, MD, PhD.
Introduction
Recent advances on next-generation sequencing (NGS) and bioinformatics analyses have contributed to an increased understanding of the genetic architecture of Parkinson’s disease (PD). Global international initiatives incorporating underrepresented non-European populations are ongoing with promising discoveries to be translated into novel therapies. Prof. Singleton, Prof. Blauwendraat and Prof. Mata discuss our current understanding of the genetic architecture of PD, the importance of global initiatives in diverse populations including historically underrepresented populations, as well as next directions in the field of PD genomics.
1. What is the current state of knowledge of the genetic architecture of Parkinson's disease?
Andy Singleton:
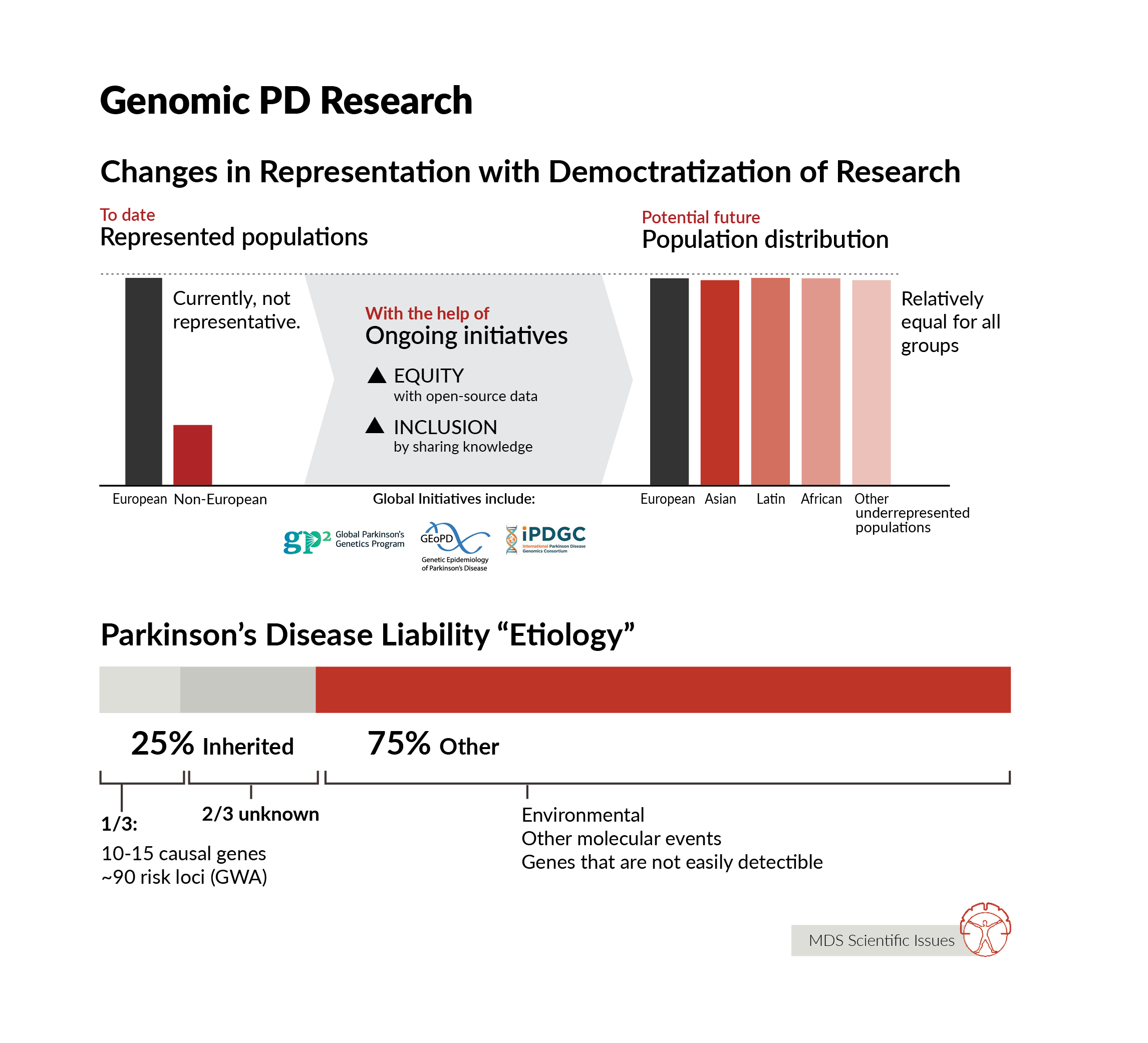
We know so much more about the genetic architecture of PD than we did even a few years ago. We know that there is a spectrum of genetic risk - where at the extreme ends there are individuals whose disease risk is almost entirely driven by genetics, usually a rare disease-causing mutation, and at the same time there are individuals with relatively minor genetic contribution to disease. Most early work in PD genetics focused on identifying rare mutations that drive disease that runs in families - and we have learnt a huge amount from identifying these mutations and understanding what they do. In the last 15 years or so, we have also made significant inroads into understanding the basis of disease in people with PD who do not carry a single, highly penetrant mutation. We have identified ~90 genetic regions (loci) that contain common genetic variants that impart risk for disease. Individually these are small, but collectively they can combine to impart significant risk. We also know that there is a lot left to find. On average, the detectable heritable component of disease accounts for ~25% of a person’s liability for disease - or to put this another way - for the average person with PD, 25% of the reason they have disease is down to detectable heritable influences. So far, we know the location of about ⅓ of this genetic component of disease. Finding more risk loci improves our capacity to make predictions about disease risk, but also provides more windows into the etiology of disease, more information about the genetic basis and subsequent mechanisms of disease risk. Notably, if there is an ~25% detectible heritable component to disease, what accounts for the remaining disease liability? Surely, environment, perhaps stochastic (or at least unmeasurable) molecular events, and likely some, as yet, genetic changes that are not easily detectable using heritability analysis. So, there is clearly much to do. Beyond finding more genetic risk, there is one prominent gap in knowledge: an understanding of the genetic architecture of PD in ancestrally diverse populations. The vast majority of work has been done in Northern European ancestry groups. This is simply not good enough - in my view genetics is the gateway to finding the right therapeutic targets, and in applying therapeutics against those targets in the right patients and the right time. If we are to treat this global disease, we therefore need to understand the genetic basis of this disease in the context of global populations.
Ignacio Mata:
We know that genetics play a role, both in familial as well as sporadic forms of the disease. And this role is quite complex with a spectrum of variants from very rare highly penetrant to very common and very small effects. There are more than 20 genes associated with familial forms of PD, although only 7-8 are bona fide PD genes where segregation has been proven in several independent families. These only explain a small number of families in the world. Some of these same genes also have common variants that play a role in sporadic disease risk. For disease risk, there are close to 100 variants in more than 75 genes that seem to have an effect (small), and when these are added up in the form of a polygenic risk score (PRS), these can explain around 20-30% of the heritable risk of PD. Thus, there is still a lot to be learn, especially for non-European populations as most of the studies to date have been performed in individuals of European or Asian populations. The few studies in Asian populations and our study in Latinos have suggested that perhaps the genetic architecture of PD is not too different between different ancestral groups, thus the differences we observe in incidence of the disease might be caused by different environmental factors, which are also known to play a role in the disease etiology.
Cornelis Blauwendraat:
After more than two decades of genetic research of PD we know quite a bit, but as always there is a lot more to discover. We know that rare mutations in about 10-15 genes can be the cause of disease and often that there are clear clinical differences among these carriers of mutations even within the same families with large ranges of age at onset, reduced penetrance and other clinical measures. Then we also know that common DNA variants can have an effect on disease and we have identified about 90 of these common risk DNA variants. One key thing that we are lacking is diversity in all of these genetic findings, which Andy and Ignacio have already highlighted. The vast majority of genetic research is performed in European ancestry individuals, which is roughly ~15% of the world population. So clearly that is an area we all need to invest in and likely there are many interesting things to discover there.
2. Why are participants and researchers from “non- European” underrepresented populations important for studying the genetics of Parkinson's disease?
Andy Singleton:
I discussed part of the rationale for conducting genetic research in ancestrally diverse populations in the previous question - simply, to treat a globally relevant disease, we need to understand the basis of this disease globally. Beyond the simple and persuasive argument that we should be aiming to treat disease in populations equitably, there are also some compelling scientific reasons to understand the basis of disease in ancestrally diverse groups. We can learn an enormous amount by comparing genetics across populations. First, doing so allows us to fine map the regions that contain risk variants, reducing the physical space in the genome in which we are searching for the underlying risk variants, or effector gene/mechanism quickly and efficiently. Second, combining work across populations allows us to define how and why diseases may differ between groups. We see considerable differences in risk, presentation, and progression of disease between groups even with the same underlying disease-causing mutation - understanding the basis of these differences sheds light on disease modifying mechanisms. Third, we can begin to identify populations that are enriched for particular genetic risk factors or causes, and plausibly prioritize these populations for clinical trials that use therapeutics aimed at their particular cause of disease.
Cornelis Blauwendraat:
We need to make Parkinson’s disease research and of course also genetics globally relevant and as mentioned earlier at the moment we are lacking there. Most of the previous studies have been focused on European ancestry populations and we know very little of the genetics of PD of other populations. Including participants of underrepresented populations in future genetic studies will shed light on how genetic risk affects disease in other ancestries, likely there will be some overlap, but also there will be clear differences. Importantly, researchers from these underrepresented populations need to drive and lead this work which is a crucial step in making the genetics of PD globally relevant.
Ignacio Mata:
Genomics in general are failing at diversity, with more than 80% of all individuals participating in genetic studies being from European ancestry, and large populations like Africans or Latinos being less than 2% (combined). In PD this is very similar, with large projects like PPMI (Parkinson's Progression Markers Initiative) and PDBP only having less than 6% of their cohorts of non-European ancestry (this includes Asian-Americans, African-Americans, Latinos, Pacific Islanders, etc.). This creates a large gap in knowledge as well as increase health disparities in many countries where these individuals are a minority and are usually not only underrepresented but also underserved. Thus, it is critical that individuals of all ancestries take part in research studies, especially genetic studies, to be able to better understand the factors that are important for the development of PD in all individuals as well as in specific populations.
3. What are the main ongoing or planned global genomic initiatives for Parkinson's disease?
Andy Singleton: Over the years there has been a few large efforts aimed at understanding the genetic basis of disease. The International Parkinson’s Disease Genomics Consortium (IPDGC) and Genetic Epidemiology of Parkinson’s disease (GEoPD) effort are two such groups, with the latter focusing on investigator lead research projects and the former enjoying success in the space of genetic risk factor identification using large scale genome wide efforts. Most recently, the Global Parkinson’s Genetics Program (GP2, https://gp2.org/) was formed with the support of the Aligning Science Across Parkinson’s Initiative (ASAP, https://parkinsonroadmap.org/), and in partnership with the Michael J Fox Foundation for Parkinson’s Disease Research (MJFF). GP2 aims to create and support a global collaborative network of investigators interested in understanding the genetic basis of disease in worldwide populations and in making this knowledge available and actionable. GP2 works with ~200 cohorts/sites around the world, including established consortia such as IPDGC Africa, IPDGC Asia, the Latin American Research consortium on the GEnetics of Parkinson's Disease (LARGE-PD), Luxembourg-German-Indian Alliance on Neurodegenerative diseases and Therapeutics (Lux-GIANT), and many others. GP2 is already the largest global effort in the PD genetics space.
Ignacio Mata:
There are many ongoing large genomic initiatives, mostly in Europe and the US. In the last few years a few were born in some parts of Asia (mainland China and Japan), and Latin America, and small efforts in other parts of the world. Some of these efforts were coordinated representing different countries, like LARGE-PD (Latin American Research consortium on the Genetics of PD) in Latin America, IPDGC (International PD Genetic Consortium) mostly in Europe and the US with branches in Asia (IPDGC-Asia) and Africa (IPDGC-Africa), GEo-PD (Genetic Epidemiology of Parkinson’s Disease) including sites in 30 different countries and Lux-GIANT (Luxemburg-German-Indian Alliance) with groups in Europe and India. All these efforts were both trying to identify and characterize PD families to understand the role of known PD genes in these regions, as well as identify novel genes, and also build case/controls series that can be used to study the genetic architecture of PD in these populations. As mentioned previously, in terms of numbers there is still a large bias towards European ancestry individuals. Most of these efforts were created in many cases without funding, and are now being supported mainly by foundations in the US like MJFF and ASAP-GP2.
Cornelis Blauwendraat:
Not much to add, both Andy and Nacho said it all I think.
4. What are the goals of the Global Parkinson’s Genetics Program "GP2" and which main milestones have been achieved so far?
Andy Singleton:
There are a series of interconnected goals within GP2. GP2 aims to accelerate our understanding of the genetic basis of PD by generating, analyzing, and disseminating results from genetic information generated in more than 150,000 volunteers from around the world. GP2 will identify new causes of disease, dramatically increase our understanding of the genetic basis of typical disease, help identify and understand the genetic basis of not only risk, but progression, onset, comorbid conditions, response to treatment, and myriad other components of PD. GP2 aims to do this in ancestrally diverse populations and in order to do so has created a global collaborative group of investigators. GP2 has spent a large amount of time creating the operational infrastructure to support its work, providing studentships, sabbaticals, training modules, compute infrastructure, and expertise to be able to quickly, effectively, and equitably make new discoveries. At the most recent count, more than 160,000 samples are in the GP2 pipeline, and the November GP2 data release will include data from almost 20,000 subjects. Although GP2 has already made some exciting discoveries, particularly looking within and across underrepresented populations, the next period will see an acceleration in discovery science in GP2.
Cornelis Blauwendraat:
With GP2 we really want to bring PD genetics to the next level and again make PD globally relevant. Over the past two and a half years we have been busy with laying the foundation for GP2 and now we are in the production phase. I think some of the main milestones achieved so far are setting up a world-wide training network with amazing online courses, generating and sharing data on a very large scale and finally inclusion of many worldwide participants and researchers. Overall, the path forward for GP2 is clear and we are very looking forward to the coming years.
Ignacio Mata:
I think the main goal of GP2 is unifying all these efforts mentioned above to provide the support and the infrastructure to better understand the genetic architecture of PD in all populations. And doing so with a very collaborative and open-science approach, sharing knowledge and data to really move the field forward. Also to create an environment of equity and inclusion, there are also a lot of resources targeted to those working in underrepresented/underserved communities. These resources not only include funding, but also much needed training opportunities so researchers in these regions can be active members of the GP2 community.