
Date: May 2024
Authors: Omar El-Agnaf, PhD; Ilham Abdi, PhD; Nobutaka Hattori, MD, PhD, FANA; Tiago Outeiro, PhD.
Editors: Lorraine Kalia, MD, PhD; Daniela Berg, MD, PhD; Jeffery H. Kordower, PhD

Introduction
Seed amplification assays (SAAs), also known as real-time quaking-induced conversion (RT-QuIC) or protein misfolding cyclic amplification (PMCA), were originally developed for the detection of the infectious form of prion protein in Creutzfeldt-Jakob disease (CJD) (Figure 1).1 With the successful development of SAAs as a diagnostic test for CJD,2 SAAs were adapted for the detection of disease-associated α-synuclein (α-syn). Some changes to the assay protocol included lowering the buffer acidity and temperature, with one of the most crucial changes being the addition of beads during the quaking process. α-Syn SAAs first reported success in the detection of aggregated α-syn in dementia with Lewy bodies (DLB) and Parkinson’s disease (PD) patients in 2016. Since then, the assay has undergone replication across the world in hopes of improving the sensitivity and specificity for synucleiopathy detection within various biofluids and tissues. In 2023, researchers using a modified α-syn SAA were the first to report differentiation between PD and MSA patients which is a promising next step for α-syn SAA development.9 The discussion below dives further into the current state of α-syn application with insight from experts in the field on the practical applications, crucial developments and future hopes for α-syn SAAs.
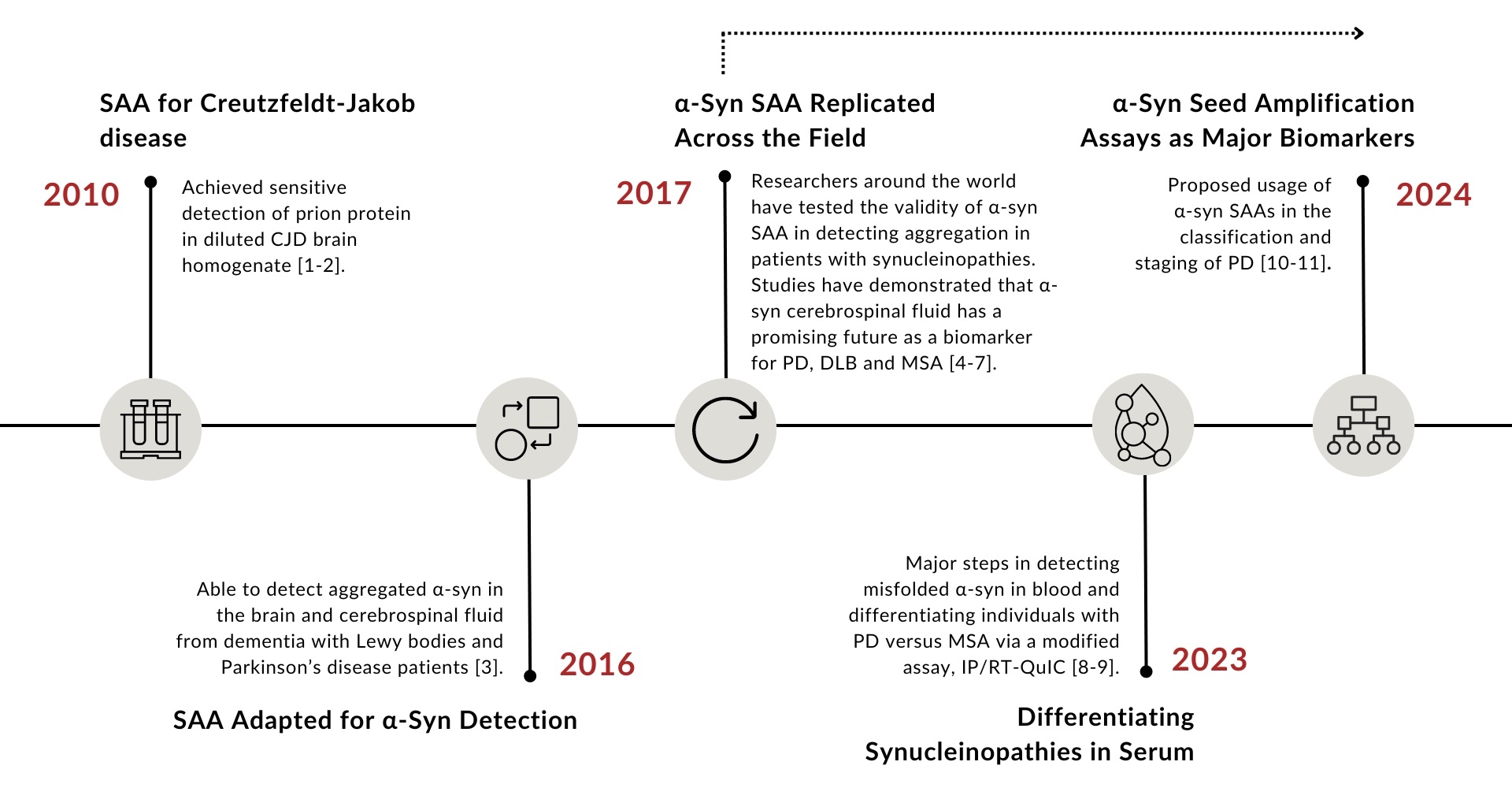
Figure 1: Historical timeline of α-syn seed amplification assay development.
| |
Article Overview
Jump to:
|
How are SAAs performed in the lab and what do they measure? What biomaterial is currently considered useful?
Prof. Outeiro:
α-Syn SAAs are performed by mixing human-derived material with recombinantly-prepared, monomeric α-syn (used as substrate), in a 96-well plate, with agitation and at 37ºC. Presently, cerebrospinal fluid (CSF) and blood are the biomaterials considered most useful, but other biomaterials such as saliva, urine, skin homogenates, nasal swabs or feces might also prove useful in the future, as new versions of the protocols emerge (Figure 2).
Dr. El-Agnaf & Dr. Abdi:
Proteinopathies involve the pathological aggregation of misfolded proteins, which can act as seeds to promote the misfolding and aggregation of the same native proteins.12 In the case of synucleinopathies, a type of proteinopathy, the aggregation of α-syn protein is a hallmark of the disease. There are three main types of synucleinopathies: PD, dementia with Lewy bodies (DLB), and multiple system atrophy (MSA) with the first two being principally a neuronal synucleinopathy and the latter being primarily an oligodendroglial synucleinopathy.13
SAAs are specifically designed to detect these aggregates with high sensitivity and specificity. They exploit this seeding mechanism by mixing a small amount of biological sample containing misfolded α-syn, or “α-syn seeds”, with a large excess of their soluble, monomeric α-syn.14 The amplification process involves cycles of incubation and agitation or sonication to promote the rapid multiplication of these seeds. This interaction facilitates the amplification of the misfolded protein aggregates, allowing for the detection of very low levels of pathological proteins. The aggregation of α-syn is monitored over time, typically by measuring the fluorescence intensity of a dye, Th-T, added that binds specifically to the formed amyloid fibrils containing β-sheet structures “aggregates”. The increase in fluorescence over time is indicative of the formation of α-syn aggregates. The kinetics of the reaction, reflected in the growth curve of fluorescence intensity, can provide information on the seeding potency of the misfolded proteins present in the original biological sample. 3,15
SAA work done for synucleinopathies, in particular PD, has extensively progressed over the last decade with the development of SAAs for various biomaterials.5,16 The most commonly used and informative biomaterial is CSF due to its close contact with the brain.17 The inclusion of diverse biofluids and tissues in SAA has been a significant focus in recent studies with many expanding the scope to include olfactory mucosa and skin tissues, reporting promising results that underline the assay's adaptability and potential in enhancing diagnostic precision and convenience.18,19 More recent work has looked at the use of SAA by incorporating an immunoprecipitation step which isolates the misfolded α-syn from the blood first then applying it to SAA.9
Dr. Hattori:
It is used for early diagnosis of PD and differential diagnosis. The most useful is aimed at general testing, and we believe it is important to measure it in blood. The SAA has finally "laid the foundation for the biological diagnosis of PD," and it has made it possible to detect the disease even before any clinical or physical changes are detected. 9,21 However, the collection of CSF or skin biopsy is more burdensome than conventional testing methods, and the development of blood tests and other less burdensome methods may be necessary to spread the use of α-syn SAA as a clue for detecting PD.
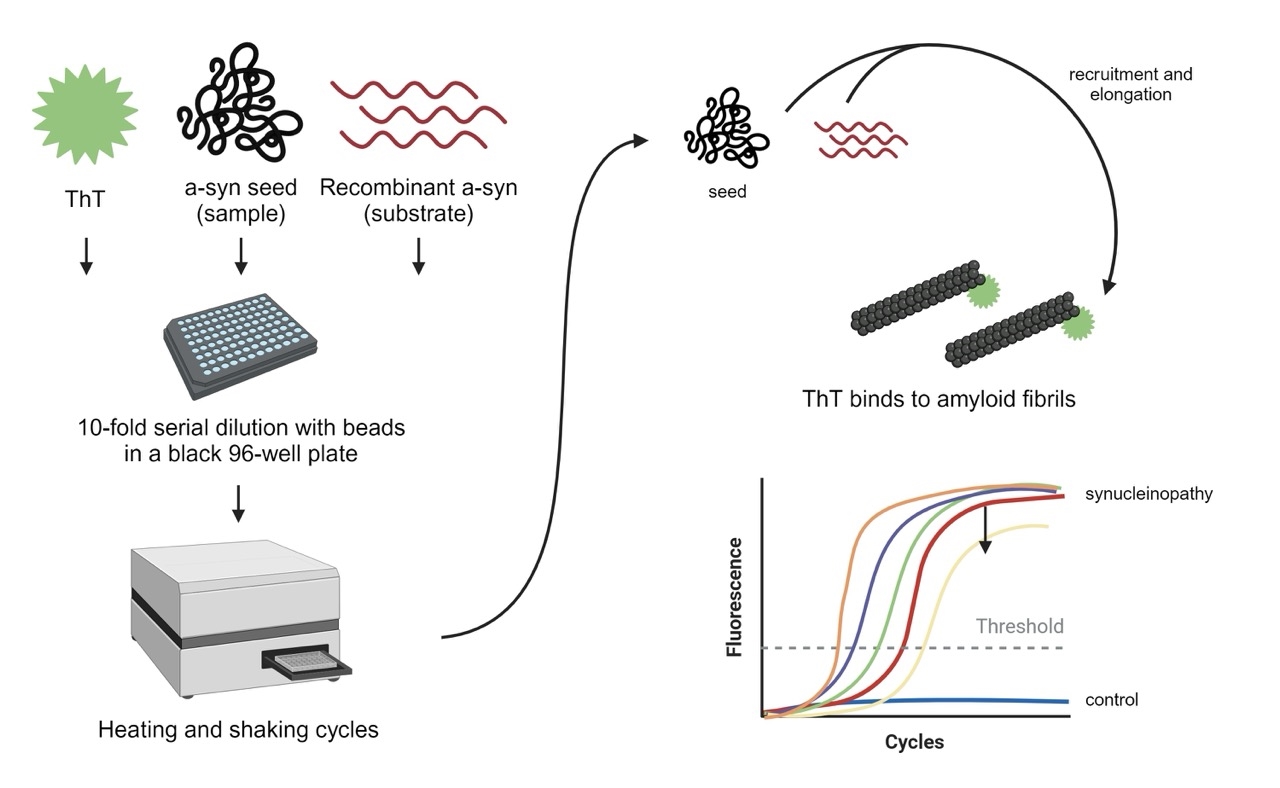
Figure 2: Schematic of the α-syn seed amplification assay. Created using Biorender.com.
What is the current role of SAAs in clinical practice? How should they be applied for people with RBD, people with established PD and people with parkinsonism with a diagnosis?
Dr. El-Agnaf & Dr. Abdi:
SAAs have emerged as important tools in clinical research of PD and related disorders. While they show great promise in research environments, SAAs have not yet achieved approval for clinical diagnostic use. However, a significant development in this field is the commercial availability of the SYNTap® Biomarker Test in the United States. This test, developed by Amprion, a pioneer in SAA technology, marks a substantial step forward in the early detection of PD and related disorders. Although not universally approved for clinical diagnostics, some clinics, like the Mayo Clinic, have begun to incorporate the SYNTap test as a supplementary tool in their diagnostic processes.20 This usage highlights the growing recognition of SAA's potential to enhance clinical understanding and patient care in neurodegenerative diseases.
The milestone Parkinson’s Progression Markers Initiative (PPMI) study by Siderowf et al. stands out as the largest analysis so far utilizing SAA, marking a significant leap in our understanding of its diagnostic performance. Involving a comprehensive 1123 participants, the study demonstrated a sensitivity of 87.7% and specificity of 96.3% for PD and highlighted its role in understanding the disease’s molecular heterogeneity and identifying prodromal individuals.21
SAAs could be particularly valuable for the early detection of α-syn aggregates in individuals at risk of developing synucleinopathies, such as those with REM sleep behaviour disorder (RBD) or hyposmia, as evidenced by several studies.21-24 Identifying these aggregates early could help in stratifying risk levels for developing PD or related disorders, thereby facilitating closer monitoring and early intervention strategies. The PPMI study highlighted SAA's capacity to distinguish PD from healthy controls and further refine diagnosis among those with parkinsonism symptoms, including those with and without genetic variants associated with PD. SAA also has the ability to distinguish between PD, DLB, and MSA from non-synucleinopathies parkinsonisms, such as progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD).25 This aids in the accurate diagnosis and appropriate management of these conditions. Further research and clinical validation in more longitudinal and diverse neurodegenerative disorder cohorts are necessary for accurate differential diagnosis and to understand the disease's underlying pathology. This would allow SAAs integration into routine clinical practice and provide critical guidance for treatment decisions.
Prof. Outeiro:
SAAs are valuable in order to determine the presence of seeding capability in individuals. According to recent data, it seems that RBD individuals already display SAA positive signal even if they do not display clinical features of the motor-phase of PD. SAAs can be very useful to classify individuals according to their α-syn status.
Dr. Hattori:
Concerning RBD patients, the consensus is that they are a risk group for synucleinopathy. Therefore, verifying how soon phenoconversion occurs after a positive result using SAA combined with immunoprecipitation is important. Once this is verified, the timing of early intervention will be determined. We also believe that in established PD patients, it will be possible to determine the relationship between SAA results and the rate of progression of clinical symptoms. As a validated test, it can differentiate Parkinsonisms, which are not a synucleinopathy. Furthermore, clinical trials targeting only synucleinopathy will be realized, and more precision medicine will be realized.
|
IMPORTANT CAUTION: SAAs are currently being used in clinical research and have yet to be verified as a diagnostic tool for prodromal PD. Please refer to these resources for more information about the ethical challenges of using SAAs in clinical practice.
Patients' views on the ethical challenges of early Parkinson disease detection. Schaeffer E, Rogge A, Nieding K, Helmker V, Letsch C, Hauptmann B, Berg D.Neurology. 2020 May 12;94(19):e2037-e2044. doi: 10.1212/WNL.0000000000009400.
Risk Disclosure in Prodromal Parkinson's Disease. Schaeffer E, Toedt I, Köhler S, Rogge A, Berg D.Mov Disord. 2021 Dec;36(12):2833-2839. doi: 10.1002/mds.28723.
|
What are the most promising developments for this methodology on the horizon? What is necessary to accomplish these developments?
Dr. Hattori:
Differentiation within synucleinopathy is now possible. We believe it is crucial to establish a more straightforward test method, if possible. Also, since specific synuclein seeds exist for each synucleinopathy, we can say that the production of disease-specific synuclein seed antibodies will be available, leading to the development of disease-specific antibody therapies.
Dr. El-Agnaf & Dr. Abdi:
The advantages of SAAs are numerous. They provide a higher sensitivity compared to other diagnostic methods and can detect misfolded proteins at early disease stages, potentially aiding in earlier diagnosis and intervention.19,26 The applicability of the technique with a variety of biological samples also presents a valued advantage.18,19,27 However, despite their potential, SAA is not without limitations. SAA has primarily been utilized in a qualitative manner to detect the presence of misfolded α-syn aggregates generating binary results of positive or negative for α-syn seeds. Quantifying α-syn seeds in biological samples would provide valuable and accurate insights for the diagnosis and monitoring of synucleinopathies. In efforts to provide a more direct method of quantifying α-syn seeds, our group recently established a novel approach combining SAA and aggregate-specific immunoassay to provide a measurable test readout showing successful direct quantification seeded α-syn in CSF samples.28 While this approach robustly quantified SAA products from PD and DLB with high specificity and sensitivity, the methodological process as a whole is complex due to the running of two separate assays to generate final results. Development of a more simplified approach using the same concept would be of great value.
The idea of incorporating immunoassay with SAA has been further explored by our own group and others. In our work, we have revamped the approach of using aggregate-specific immunoassay alongside the SAA by developing a single assay that effectively amplifies α-syn seeds and quantifies them at the same time (manuscript submitted for publication). Also, there are different diagnostic assays, recently developed by researchers from Brigham and Women’s Hospital and the Wyss Institute for Biologically Inspired Engineering at Harvard University, referred to as digital seed amplification assays (dSAAs), that operate by isolating single ⍺-synuclein aggregates in engineered microcompartments and grown into larger fluorescent aggregates that become easily detectible and quantifiable.22
These efforts focused on refining SAAs to be not just qualitative but quantitative tools, capable of accurately measuring the amount of disease-specific protein aggregates, are promising developments that should be further supported. Successfully validating these developments on a larger scale could mark a significant milestone in integrating it into clinical practice and clinical trials achieving early and accurate diagnosis, ultimately enhancing patient outcomes.
Prof. Outeiro:
Increasing the sensitivity of the assay, making SAAs quantitative, and adapting SAAs to more accessible human biomaterials would be important developments. To accomplish this, we need additional research to identify assay conditions and substrates that improve the assay.
How do you envision the applications of SAAs in future clinical practice and clinical trials?
Prof. Outeiro:
I envision that SAA status will be instrumental for classifying individuals for clinical trials, and also, possibly, as a marker of target engagement.
Dr. Hattori:
We can say that it can be applied first to precision medicine. Identifying patients at higher risk of developing the disease is also possible as a physical examination of the risk group. As a result, it will be helpful for preventive medicine.
Dr. El-Agnaf & Dr. Abdi:
Following the development and validation of a quantitative SAA, its potential applications can result in more efficient clinical trials, and the development of more effective, personalized treatment strategies.
SAA could enable the detection of PD, DLB, and MSA before the onset of clinical symptoms, allowing for interventions at stages when therapies are more likely to be effective. This early diagnostic capability would not only transform patient care strategies but also refine patient selection criteria for clinical trials, ensuring that participants are accurately diagnosed and at the right stage of disease progression for potential treatments to have the maximum impact. Furthermore, by quantifying disease-specific protein aggregates, SAAs could serve as biomarkers for tracking disease progression and response to therapy, offering a dynamic tool for monitoring patient health over time. In clinical trials, this could dramatically enhance the evaluation of therapeutic efficacy, providing clear, quantifiable endpoints that reflect changes in the underlying pathology of the disease.
References
-
Wilham, J. M., Orrú, C. D., Bessen, R. A., Atarashi, R., Sano, K., Race, B., Meade-White, K. D., Taubner, L. M., Timmes, A., & Caughey, B. (2010). Rapid end-point quantitation of prion seeding activity with sensitivity comparable to bioassays. PLoS pathogens, 6(12), e1001217. https://doi.org/10.1371/journal.ppat.1001217
-
Atarashi, R., Satoh, K., Sano, K., Fuse, T., Yamaguchi, N., Ishibashi, D., Matsubara, T., Nakagaki, T., Yamanaka, H., Shirabe, S., Yamada, M., Mizusawa, H., Kitamoto, T., Klug, G., McGlade, A., Collins, S. J., & Nishida, N. (2011). Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nature medicine, 17(2), 175–178. https://doi.org/10.1038/nm.2294
-
Fairfoul, G., McGuire, L. I., Pal, S., Ironside, J. W., Neumann, J., Christie, S., Joachim, C., Esiri, M., Evetts, S. G., Rolinski, M., Baig, F., Ruffmann, C., Wade-Martins, R., Hu, M. T., Parkkinen, L., & Green, A. J. (2016). Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Annals of clinical and translational neurology, 3(10), 812–818. https://doi.org/10.1002/acn3.338
-
Bongianni, M., Ladogana, A., Capaldi, S., Klotz, S., Baiardi, S., Cagnin, A., Perra, D., Fiorini, M., Poleggi, A., Legname, G., Cattaruzza, T., Janes, F., Tabaton, M., Ghetti, B., Monaco, S., Kovacs, G. G., Parchi, P., Pocchiari, M., & Zanusso, G. (2019). α-Synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Annals of clinical and translational neurology, 6(10), 2120–2126. https://doi.org/10.1002/acn3.50897
-
Rossi, M., Candelise, N., Baiardi, S., Capellari, S., Giannini, G., Orrù, C. D., Antelmi, E., Mammana, A., Hughson, A. G., Calandra-Buonaura, G., Ladogana, A., Plazzi, G., Cortelli, P., Caughey, B., & Parchi, P. (2020). Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta neuropathologica, 140(1), 49–62. https://doi.org/10.1007/s00401-020-02160-8
-
Brockmann, K., Quadalti, C., Lerche, S., Rossi, M., Wurster, I., Baiardi, S., Roeben, B., Mammana, A., Zimmermann, M., Hauser, A. K., Deuschle, C., Schulte, C., Waniek, K., Lachmann, I., Sjödin, S., Brinkmalm, A., Blennow, K., Zetterberg, H., Gasser, T., & Parchi, P. (2021). Association between CSF alpha-synuclein seeding activity and genetic status in Parkinson's disease and dementia with Lewy bodies. Acta neuropathologica communications, 9(1), 175. https://doi.org/10.1186/s40478-021-01276-6
-
Poggiolini, I., Gupta, V., Lawton, M., Lee, S., El-Turabi, A., Querejeta-Coma, A., Trenkwalder, C., Sixel-Döring, F., Foubert-Samier, A., Pavy-Le Traon, A., Plazzi, G., Biscarini, F., Montplaisir, J., Gagnon, J. F., Postuma, R. B., Antelmi, E., Meissner, W. G., Mollenhauer, B., Ben-Shlomo, Y., Hu, M. T., … Parkkinen, L. (2022). Diagnostic value of cerebrospinal fluid alpha-synuclein seed quantification in synucleinopathies. Brain : a journal of neurology, 145(2), 584–595. https://doi.org/10.1093/brain/awab431
-
Kluge, A., Bunk, J., Schaeffer, E., Drobny, A., Xiang, W., Knacke, H., Bub, S., Lückstädt, W., Arnold, P., Lucius, R., Berg, D., & Zunke, F. (2022). Detection of neuron-derived pathological α-synuclein in blood. Brain : a journal of neurology, 145(9), 3058–3071. https://doi.org/10.1093/brain/awac115
-
Okuzumi, A., Hatano, T., Matsumoto, G., Nojiri, S., Ueno, S. I., Imamichi-Tatano, Y., Kimura, H., Kakuta, S., Kondo, A., Fukuhara, T., Li, Y., Funayama, M., Saiki, S., Taniguchi, D., Tsunemi, T., McIntyre, D., Gérardy, J. J., Mittelbronn, M., Kruger, R., Uchiyama, Y., … Hattori, N. (2023). Propagative α-synuclein seeds as serum biomarkers for synucleinopathies. Nature medicine, 29(6), 1448–1455. https://doi.org/10.1038/s41591-023-02358-9
-
Simuni, T., Chahine, L. M., Poston, K., Brumm, M., Buracchio, T., Campbell, M., Chowdhury, S., Coffey, C., Concha-Marambio, L., Dam, T., DiBiaso, P., Foroud, T., Frasier, M., Gochanour, C., Jennings, D., Kieburtz, K., Kopil, C. M., Merchant, K., Mollenhauer, B., Montine, T., … Marek, K. (2024). A biological definition of neuronal α-synuclein disease: towards an integrated staging system for research. The Lancet. Neurology, 23(2), 178–190. https://doi.org/10.1016/S1474-4422(23)00405-2
-
Höglinger, G. U., Adler, C. H., Berg, D., Klein, C., Outeiro, T. F., Poewe, W., Postuma, R., Stoessl, A. J., & Lang, A. E. (2024). A biological classification of Parkinson's disease: the SynNeurGe research diagnostic criteria. The Lancet. Neurology, 23(2), 191–204. https://doi.org/10.1016/S1474-4422(23)00404-0
-
Bayer TA. Proteinopathies, a core concept for understanding and ultimately treating degenerative disorders? Eur Neuropsychopharmacol [Internet]. 2015 May 1 [cited 2024 Feb 18];25(5):713–24. Available from: https://pubmed.ncbi.nlm.nih.gov/23642796/
-
McCann H, Stevens CH, Cartwright H, Halliday GM. α-Synucleinopathy phenotypes. Parkinsonism Relat Disord. 2014 Jan 1;20(SUPPL.1):S62–7.
-
Concha-Marambio L, Pritzkow S, Shahnawaz M, Farris CM, Soto C. Seed amplification assay for the detection of pathologic alpha-synuclein aggregates in cerebrospinal fluid. Nature Protocols 2023 18:4 [Internet]. 2023 Jan 18 [cited 2023 Nov 12];18(4):1179–96. Available from: https://www.nature.com/articles/s41596-022-00787-3
-
Shahnawaz M, Tokuda T, Waraga M, Mendez N, Ishii R, Trenkwalder C, et al. Development of a Biochemical Diagnosis of Parkinson Disease by Detection of α-Synuclein Misfolded Aggregates in Cerebrospinal Fluid. JAMA Neurol [Internet]. 2017 Feb 1 [cited 2023 Sep 24];74(2):163–72. Available from: https://jamanetwork.com/journals/jamaneurology/fullarticle/2588684
-
Shahnawaz M, Mukherjee A, Pritzkow S, Mendez N, Rabadia P, Liu X, et al. Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature [Internet]. 2020 Feb 13 [cited 2023 Nov 23];578(7794):273–7. Available from: https://pubmed.ncbi.nlm.nih.gov/32025029/
-
El-Agnaf OMA, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ, et al. α-Synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. The FASEB Journal [Internet]. 2003 Oct 1 [cited 2024 Feb 18];17(13):1–16. Available from: https://onlinelibrary.wiley.com/doi/full/10.1096/fj.03-0098fje
-
Kuzkina A, Bargar C, Schmitt D, Rößle J, Wang W, Schubert AL, et al. Diagnostic value of skin RT-QuIC in Parkinson’s disease: a two-laboratory study. npj Parkinson’s Disease 2021 7:1 [Internet]. 2021 Nov 15 [cited 2023 Nov 22];7(1):1–11. Available from: https://www.nature.com/articles/s41531-021-00242-2
-
De Luca CMG, Elia AE, Portaleone SM, Cazzaniga FA, Rossi M, Bistaffa E, et al. Efficient RT-QuIC seeding activity for α-synuclein in olfactory mucosa samples of patients with Parkinson’s disease and multiple system atrophy. Transl Neurodegener [Internet]. 2019 Aug 8 [cited 2023 Nov 22];8(1):1–14. Available from: https://translationalneurodegeneration.biomedcentral.com/articles/10.1186/s40035-019-0164-x
-
Mayo Clinic. Parkinson’s test (a-Synuclein seed amplification assay) - Mayo Clinic [Internet]. 2023 [cited 2024 Feb 18]. Available from: https://www.mayoclinic.org/tests-procedures/parkinsons-testing-alpha-synuclein-seed-amplification/about/pac-20556191
-
Siderowf A, Concha-Marambio L, Lafontant DE, Farris CM, Ma Y, Urenia PA, Nguyen H, Alcalay RN, Chahine LM, Foroud T, Galasko D, Kieburtz K, Merchant K, Mollenhauer B, Poston KL, Seibyl J, Simuni T, Tanner CM, Weintraub D, Videnovic A, Choi SH, Kurth R, Caspell-Garcia C, Coffey CS, Frasier M, Oliveira LMA, Hutten SJ, Sherer T, Marek K, Soto C; Parkinson's Progression Markers Initiative. Assessment of heterogeneity among participants in the Parkinson's Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol. 2023 May;22(5):407-417. doi: 10.1016/S1474-4422(23)00109-6. PMID: 37059509; PMCID: PMC10627170.
-
Gilboa T, Swank Z, Thakur R, Gould RA, Ooi KH, Norman M, et al. Toward the quantification of α-synuclein aggregates with digital seed amplification assays. Proc Natl Acad Sci U S A [Internet]. 2024 Jan 16 [cited 2024 Feb 18];121(3). Available from: https://pubmed.ncbi.nlm.nih.gov/38194461/
-
Postuma RB. Prodromal Parkinson’s disease – Using REM sleep behavior disorder as a window. Parkinsonism Relat Disord. 2014 Jan 1;20(SUPPL.1):S1–4.
-
Concha-Marambio L, Weber S, Farris CM, Dakna M, Lang E, Wicke T, et al. Accurate Detection of α-Synuclein Seeds in Cerebrospinal Fluid from Isolated Rapid Eye Movement Sleep Behavior Disorder and Patients with Parkinson’s Disease in the DeNovo Parkinson (DeNoPa) Cohort. Mov Disord [Internet]. 2023 Apr 1 [cited 2024 Feb 18];38(4):567–78. Available from: https://pubmed.ncbi.nlm.nih.gov/36781413/
-
Fernandes Gomes B, Farris CM, Ma Y, Concha-Marambio L, Lebovitz R, Nellgård B, et al. α-Synuclein seed amplification assay as a diagnostic tool for parkinsonian disorders. Parkinsonism Relat Disord [Internet]. 2023 Dec 1 [cited 2024 Feb 19];117. Available from: https://pubmed.ncbi.nlm.nih.gov/37591709/
-
Iranzo A, Fairfoul G, Ayudhaya ACN, Serradell M, Gelpi E, Vilaseca I, et al. Detection of α-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: a longitudinal observational study. Lancet Neurol [Internet]. 2021 Mar 1 [cited 2023 Sep 24];20(3):203–12. Available from: https://pubmed.ncbi.nlm.nih.gov/33609478/
-
Manne S, Kondru N, Jin H, Anantharam V, Huang X, Kanthasamy A, et al. α-Synuclein real-time quaking-induced conversion in the submandibular glands of Parkinson’s disease patients. Mov Disord [Internet]. 2020 Feb 1 [cited 2023 Nov 22];35(2):268–78. Available from: https://pubmed.ncbi.nlm.nih.gov/31758740/
-
Majbour N, Aasly J, Abdi I, Ghanem S, Erskine D, Van De Berg W, et al. Disease-Associated α-Synuclein Aggregates as Biomarkers of Parkinson Disease Clinical Stage. Neurology [Internet]. 2022 Nov 22 [cited 2023 Jun 11];99(21):E2417–27. Available from: https://pubmed.ncbi.nlm.nih.gov/36096686/