 VOLUME 28, ISSUE 2 • JUNE 2024. Full issue »
VOLUME 28, ISSUE 2 • JUNE 2024. Full issue »

Mixed pathology variant of multiple system atrophy highlights novel concept to interpret co-pathologies
What if co-pathology doesn’t alter clinical phenotype? There may be more than meets the eye when it comes to co-pathology identification.
On behalf of the authors, it is our pleasure to present a summary of a recent study on a rare mixed pathology phenotype in multiple system atrophy (MSA).1
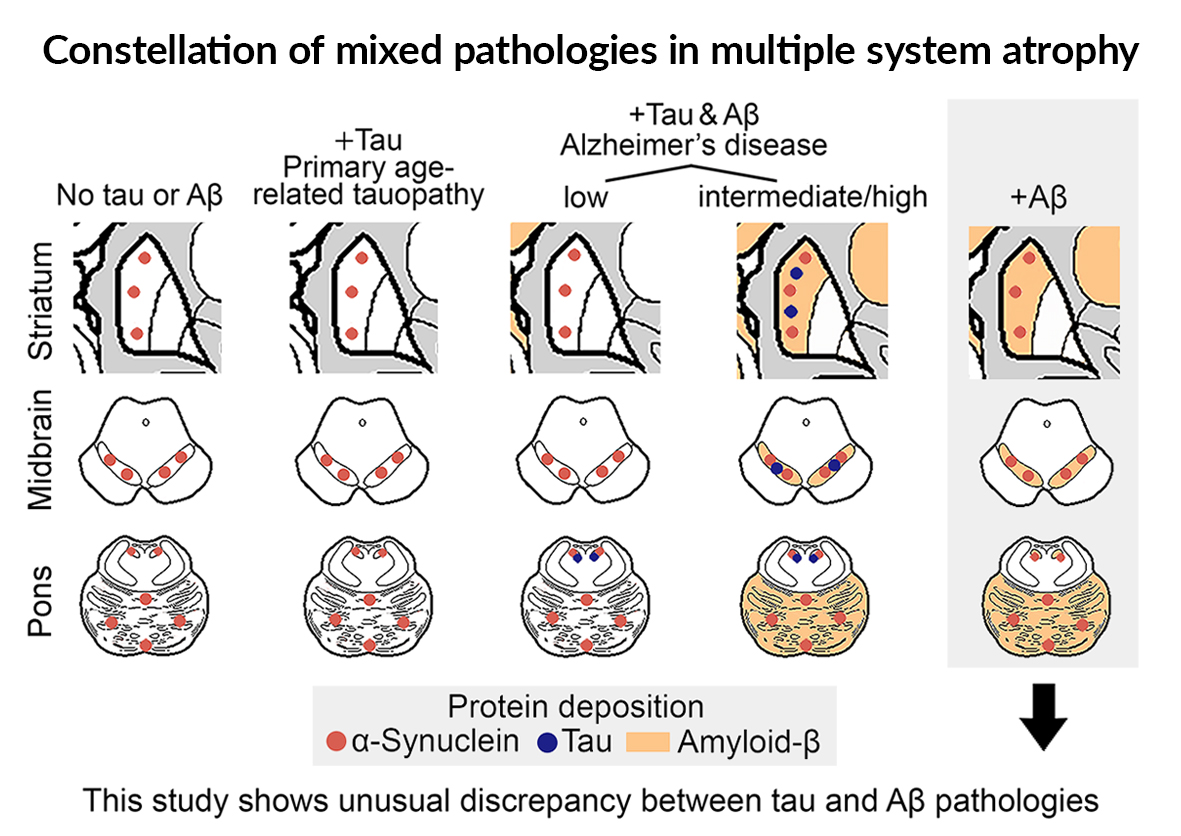
Neurodegenerative diseases are a pathologically, clinically, and genetically diverse group of disorders neuropathologically characterized by disease-specific protein aggregates in neurons and/or glia. A combination of pathological alterations such as vascular lesions and the deposition of multiple protein deposits are commonly observed, referred to as mixed pathology.2 There is ongoing discussion about the relevance of mixed pathologies on the clinical phenotype. Multiple system atrophy (MSA) is regarded as unique among neurodegenerative disorders, since most of them do not have a significant degree of co-pathology with only minimal clinical impact.2,3
Studies on mixed pathologies in MSA focus on the presence and impact of Alzheimer’s-disease related neuropathologic change (ADNC). ADNC is characterized by the concomitant presence of Amyloid-b (Ab) and tau pathology, both affecting the striatum, the substantia nigra, and cerebellum (i.e., the strategic regions in MSA) only in advanced phases and stages.2 Importantly, in primary age-related tauopathy (PART) tau pathology that is not accompanied by Ab does not involve regions beyond the medial temporal lobe. Therefore, except for advanced ADNC, which would already be associated with severe cognitive decline, a brain regional meeting for significant interaction between Ab, tau, and a-synuclein is less likely (Figure).
In contrast, in the 4 MSA cases reported,1 an unusual discrepancy between the terminal Aβ-deposition phase and incipient tangle stage, referred to as “Aβ-predominant ADNC-MSA,” was detected.1 These cases did not show a peculiar phenotype, but had more premature Aβ plaques compared to typical AD cases. α-Synuclein seeding amplification assay showed differences in the kinetics in two cases.
What is the relevance of reporting this? Imagine that you consider a-synuclein targeted therapy for a patient with MSA, and biomarker tests suggest presence of a-synuclein and concomitant advanced Ab but no tau pathology. Our study helps in the interpretation: i) the clinical course is most likely not affected by this, but ii) there is a high chance that Ab meets a-synuclein in strategic brain regions (i.e., striatum, pons, cerebellum) (Figure) and potentially influence a-synuclein misfolding or therapies targeting that.
In our opinion, emerging biomarker studies in clinical practice will allow the recognition of these cases, and the clinicians have to be informed. We propose a two-tiered approach to the interpretation of mixed pathologies not only to address the questions of whether the additional misfolded protein affects the clinical phenotype, but whether there is an anatomical overlap between the misfolded protein deposits that allow direct interaction.1
References
- Kon T, Ichimata S, Di Luca DG, et al. Multiple system atrophy with amyloid-beta-predominant Alzheimer's disease neuropathologic change. Brain Commun 2024; 6(3): fcae141.
- Forrest SL, Kovacs GG. Current Concepts of Mixed Pathologies in Neurodegenerative Diseases. Can J Neurol Sci 2023; 50(3): 329-45.
- Sekiya H, Koga S, Murakami A, et al. Frequency of Comorbid Pathologies and Their Clinical Impact in Multiple System Atrophy. Mov Disord 2024; 39(2): 380-90.
Read more Moving Along:






